Corrosive wear is defined as the damage caused by synergistic attack of wear and corrosion when wear occurs in a corrosive environment. Corrosive wear can be distinguished as Galvanic corrosion and Pitting corrosion. Galvanic corrosion (also called bimetallic corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, in the presence of an electrolyte. Pitting corrosion is a localized form of corrosion by which cavities are produced in the material. Pitting is considered to be more dangerous than uniform corrosion damage because it is more difficult to detect.
Hard materials having small percentage of chrome deposited through HP-HVOF or Plasma process provide good resistance to corrosive wear. Few of these materials can also be deposited through cladding process to achieve higher thickness with metallurgical bond. Few coatings suggested by us for applications involving Corrosive Wear are :
| Material Category | Material Code |
|---|---|
| Ceramic | - B02 |
| Alloys & Metal Blend | - C25 - C83X |
| Carbide | - DC07 - DC09 |
High temperature corrosion usually occurs at temperature above 500°C when the metal component is exposed to oxidizing atmosphere such as sulphur, oxygen or other oxidizing compounds for a long period. This type of phenomenon usually occurs in gas turbine engines and aircraft engines.
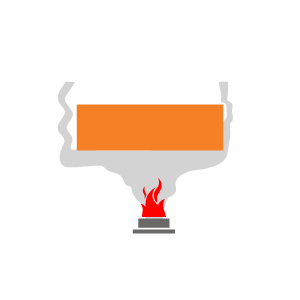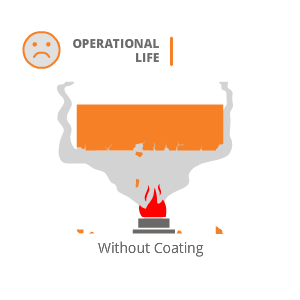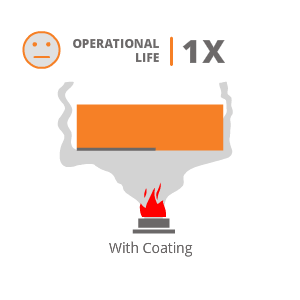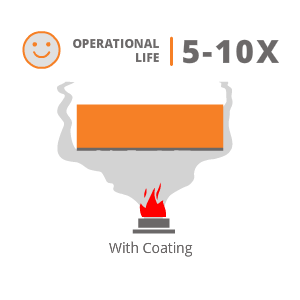
Hard material with a property to withstand high operating temperature, deposited by HP-HVOF or Plasma process will provide good resistance to high temperature corrosion resistance. Few coatings suggested by us for applications involving High Temperature Corrosive Wear are :
| Material Category | Material Code |
|---|---|
| Ceramic | - B01 - B03 |
| Alloys & Metal Blend | - C25 - C52 - C53 |
| Cermet | - D21 - D34 - D36 - D50 |